Comparative study between diclofenac and acetaminophen paediatric suppositories on perioperative pain management in orthopaedic paediatric surgeries
2 Al-Shifaa’ General Hospital, Basrah, Iraq, Email: salihkazim@yahoo.com
Received: 30-Mar-2021 Accepted Date: Apr 13, 2021 ; Published: 20-Apr-2021, DOI: 10.37532/1897-2276.2021.16(1).14
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: In the paediatric age group, in particular, post-operative pain relief is a crucial part of patient care. Besides local anesthetic agents and opioids, NSAIDs are the most widely used for this purpose. This study is to comapre the effectivess of Diclofenac sodium suppositories versus Acetaminophen suppositories in perioperative pain releif in paediatric orthopaedic patients.
Methods: A prospective double-blind randomized comparative study included. Fifty children aged one to eight years undergoing differnt orthopefic surgies. They were randomly allocated to either (Group A) treated with Diclofenac suppositories at the dose of 1 mg/kg-3 or to (Group B) treated with Acetaminophen suppositories at the dose of 15 mg/kg-20 mg/kg. Pain relief assessed by using Faces-pain score from zero to five with a score of three or above is considered as a failure of pain relief.
Results: The mean age was 4.2 +- 2.42 years, commonest age group was two years (22%=11 child). 29 (58%) patients from 50 received Diclofenac suppositories, 26 of them scored below or equal to two including 21 patients who underwent major or super-major surgeries. On the other hand, 21 (42%) patients received Acetaminophen suppositories, 20 of them scored three and above including 10 patients who underwent major or super-major surgeries, while only one patient scored two. This indicates a superior analgesic effect of Diclofenac as compared to Acetaminophen in decreasing postoperative pain (p<0.001).
Conclusion: Diclofenac sodium is better than acetaminophen in the treatment of postoperative pain in paediatric surgical orthopaedic practice.
Keywords
diclofenac, acetaminophen, paediatric suppositories, pain management, orthopaedic paediatric
Introduction
Pain may be a reflection of poor surgical interference therefore, it must be abolished or minimized, so that the child could pass through a smooth perioperative period and achieve early mobilization after surgery, reduce the length of hospital stay and ameliorate other postoperative complications, as well as avoid the dissatisfaction of the worry parents. Analgesia prescription must take into account medical, psychological, and physical condition, age, level of fear or anxiety, surgical procedure, personal preference, and response to agents given. The optimal strategy for perioperative pain control consists of multimodal therapy to minimize the need for opioids [1], and surgery may be the trigger for long-term opioid use in many patients [2]. This study analyzed postoperative pain relief in the pediatric age group who had undergone different types of orthopaedic surgeries by using two types of rectal paediatric analgesics; and assessing the pain by using a Face-pain score.
Materials and Methods
A prospective double-blind comparative study was conducted in Basra Teaching Hospital between April 2018 and November 2018 after getting approval from the research ethics committee in the hospital. It is a randomized clinical trial involved 50 children aged one to eight years who underwent different orthopaedic surgeries. One group was treated with rectal Diclofenac suppositories at 1-3 mg\kg dose (Group A) and the other group received rectal Acetaminophen at 15-20 mg \ kg dose (Group B), the randomization to either group used computer assignment. Assessment for the extent of pain was done by using Facespain score which consists of six points from 0 (no pain) to 5 (worst pain), these points reflect facial expressions of the child [3]. A score of 0, 1, or 2 was considered as good pain relief while a score of 3 or more was considered as a failure of pain relief.
According to the bodyweight of each child, suitable doses of both diclofenac and acetaminophen suppositories were packed by an independent pharmacist into two containers named A and B and marked with a reference number for later review. Both medications were used by the same manufacturer. The medications are given by an anesthesia staff member who is blinded about the type of the medication within 30 minutes pre or post-operatively. An examiner assessed the faces pain score of the child in the recovery room, after thirty minutes, one hour later, and then two-hourly until for eight hours, an average of the observations considered as the final score.
Inclusion criteria: Children from one to eight years old undergoing different orthopaedic surgeries.
Exclusion criteria
• Children less than one and more than eight years of age: those under one year old were excluded because Diclofenac is contraindicated to be given at this age, whereas those above eight years were excluded because of lack of a commercially available suppository dose suitable for older children with higher body weight
• Asthmatic patients
• Patients with a history of gastrointestinal bleeding
• Patients with renal and hepatic diseases
• History of allergy to Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Statistical Analysis
Data were analyzed using IBM SPSS software version 24, patients employed for the study, were compared in terms of age, gender, type of surgeries, time of giving the suppositories, and response to the treatment(pain relief). Chi-square test and significance level at (p-value ≤ 0.001) were used to look for any significant association between the treatment and response.
Results
Data from 50 children aged 1-8 years old were analyzed, the mean age was 4.2 ± 2.42 (Table 1). The commonest age group was two years (22%=11 child), 24 (48%) patients were boys and 26 (52%) girls. The total number of children who received Diclofenac suppositories was 29 (58.0%), while the total number of those who received Acetaminophen suppositories was 21 (42.0%). The numbers of children who received suppositories in pre-operative time were 19 (38.0%), ten of them given Diclofenac, while nine of them given Acetaminophen. The number of children who received suppositories in post-operative time were 31 (62.0%), nineteen of them given Diclofenac, while twelve of them took acetaminophen (Table 2). Sixteen patients were given treatment in moderate surgeries, 20 in major and 14 in super-major surgeries (Table 3). In twenty-six patients who received Diclofenac suppositories, the score was two or less, which indicates the higher efficacy of Diclofenac in decreasing post-operative pain, while only in one patient who received Acetaminophen the score was two.
Table 1. Age and gender distribution
| Age | Gender | Total | |
|---|---|---|---|
| Male | Female | ||
| 1-5 | 13 | 20 | 33 |
| 6-10 | 11 | 6 | 17 |
| 24 | 26 | 50 | |
Table 2.Timing of giving analgesia
| Suppositories | Timing of giving analgesia | Total | Percent | |
|---|---|---|---|---|
| Preoperative | Postoperative | |||
| Diclofenac | 10 | 19 | 29 | 0.58 |
| Acetaminophen | 9 | 12 | 21 | 0.42 |
| Total | 19 | 31 | 50 | 1 |
Table 3. Type of surgery
| Suppositories | Type of surgery | Total | ||
|---|---|---|---|---|
| Minor | Major | Super major | ||
| Diclofenac | 5 | 13 | 11 | 29 |
| Acetaminophen | 11 | 7 | 3 | 21 |
| Total | 16 | 20 | 14 | 50 |
Twenty patients who received Acetaminophen, the score was three and above this indicates the failure of Acetaminophen in decreasing postoperative pain (Table 4). Table 5 shows the correlations between the Faces Pain Score and the type of perioperative suppositories. observation of Table 5 shows that most of the patients with good pain relief (score 0,1, or 2) were those who had been treated with diclofenac suppositories, on the other hand, those who received acetaminophen suppositories had poor pain relief. Chi-square test used to look for significant association between the type of analgesic suppositories and pain score as shown in Table 6, it is evident that diclofenac suppositories were associated with significant pain relief as compared to acetaminophen (p-value < 0.00001).
Table 4. Face pain score versus type of surgery and suppositories
| Face pain score | Suppositories | Minor | Major | Super major | Total (%) |
|---|---|---|---|---|---|
| 0 | Diclofenac | 0 | 1 | 0 | 1(2%) |
| 1 | Diclofenac | 2 | 2 | 7 | 11(22%) |
| 2 | Diclofenac | 3 | 9 | 2 | 14 (28%) |
| Acetaminophen | 1 | 0 | 0 | 1 (2%) | |
| 3 | Diclofenac | 0 | 1 | 1 | 2 (4%) |
| Acetaminophen | 7 | 3 | 0 | 10 (20%) | |
| 4 | Diclofenac | 0 | 0 | 1 | 1(2%) |
| Acetaminophen | 3 | 3 | 3 | 9 (18%) | |
| 5 | Acetaminophen | 0 | 1 | 0 | 1 (2%) |
| Total | 16 | 20 | 14 | 50 (100%) | |
Table 5. Face pain score and type of suppositories
| Face pain score | Diclofenac Supp. | Acetaminophen Supp. | Total |
|---|---|---|---|
| 0 | 1 | 0 | 1 |
| 1 | 11 | 0 | 11 |
| 2 | 14 | 1 | 15 |
| 3 | 2 | 10 | 12 |
| 4 | 1 | 9 | 10 |
| 5 | 0 | 1 | 1 |
| Total | 29 | 21 | 50 |
Table 6. Association between pain relief and the analgesic agent
| Type of analgesic | Pain score | Chi square value | p value | |
|---|---|---|---|---|
| Good pain relief | Poor pain relief | |||
| Diclofenac supp. | 26 | 3 | 35.338 | <0.00001 |
| Acetaminophen sup. | 1 | 20 | ||
Discussion
Despite the plenty of methods available for post-operative pain relief, it is claimed that children are still undertreated for this very common problem with 40% of them experiencing moderate to severe pain in the study by Kozlowski LJ et al. [4-6], many studies have been carried out to evaluate for postoperative analgesia, and recommendations for children continue to evolve [7].
With advanced studies in perioperative pain, emphasis is on combined pharmacological approaches (multimodal therapy). Simultaneous use of centrally acting and peripherally acting analgesics has a better response in pain management than in either class of drug used alone [1,8].
Overall, the literature supports the use of NSAID for post-operative pain in various surgical fields, in this instance, guidance from the pain committee of the European Society for Paediatric Anaesthesiology (ESPA Pain Management Ladder Initiative) suggest basic, intermediate, and advanced pain management methods with NSAID recommended for perioperative analgesia in all steps of the ladder since they are widely available, proven to work, safe, do not require any complex monitoring, also the NSAID is familiar for most of the health staff and not requiring high-tech delivery systems [6,9]. We preferred the prior use of NSAIDs to prevent the onset of pain, contributing to psychological well-being, comfort, and improvement in the general condition of the patient.
Baer et. al [10] compared the effects of rectally administered diclofenac (12.5 mg) versus paracetamol (125 mg) both given in combination with diazepam (0.5 mg kg-1) about 20 min before surgery in 44 children scheduled for adenoidectomy (with or without myringotomy). During recovery, children in the diclofenac group needed fewer supplementary doses of intravenous pethidine than those receiving paracetamol (p<0.001), and no postoperative complications became evident, they propose that the pre-operative rectal administration of diclofenac for pain relief after adenoidectomy is safe and effective. Similar results were obtained by Tawalbeh MI et al. [11], who compared the analgesic efficacy of diclofenac sodium suppositories (1-3 mg/kg) postoperatively and paracetamol syrup (10-15 mg/kg) in 4 divided doses. Diclofenac sodium has a significant effect on decreasing postoperative pain and improved oral intake reduced nausea and vomiting, and allowed safer and earlier hospital discharge. Bhavsar MM et al [12] reached a similar conclusion supporting the longer duration of analgesia provided by rectal diclofenac in children with hydrocephaly.
In the randomized clinical trial by Mireskandari S.M. et al [13], the analgesic effects of acetaminophen, diclofenac, and their combination after cleft palate surgery were compared in 120 children (1.5 years to 5 years). Children were randomized to receive placebo, acetaminophen (40 mg/kg), diclofenac (1 mg/kg), or acetaminophen (40 mg/kg) plus diclofenac (1 mg/kg) rectally just after surgery. Acetaminophen and diclofenac were administered every 8 hours until 48 hours, meperidine was given as rescue analgesia in children with high pain scores. The need for meperidine was significantly higher in the acetaminophen group than in the diclofenac group (p < 0.05). Adverse effects were comparable among groups. Rectal acetaminophen plus diclofenac was found to be the most effective in pain control.
Chimaobi T. N et al [14] designed a level 1 evidence treatment study, used multimodal therapy involving 1 ml/kg of 0.25% caudalbupivacaine alone or combined with either 1 mg/kg rectal-diclofenac or 30 mg/kg rectal acetaminophen, 87 children intended for elective day case inguinal-herniotomy were randomly assigned to either group, they concluded that the combined treatment approach using bupivacaine with diclofenac provides a significantly more prolonged postoperative analgesia and lower pain score than the combination of bupivacaine and acetaminophen.
Conclusion
Diclofenac sodium is better than acetaminophen in the treatment of postoperative pain in paediatric surgical orthopaedic practice, we strongly recommend considering this study by clinical applications to our boys and girls in the hospitals especially in the orthopaedic units to satisfy the child and his family.
Acknowledgement
We are thankful to all the theatre staff members and the Department of Anaesthesia, we are also thankful to the surgeons for their kind cooperation and our patients and their caregivers.
Conflicts of Interest
The authors declare no conflicts of interest.
REFERENCES
- Mariano E.R.: Management of acute perioperative pain. 2011
- Mariano E.R.: Management of acute perioperative pain. 2010
- Mudumbai S.C., Oliva E.M., Lewis E.T., et al.: Time-to-cessation of postoperative opioids: a population-level analysis of the Veterans Affairs Health Care System. Pain Med. 2016;17:1732-1743
- https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1519
- Kozlowski L.J., Kost-Byerly S., Colantuoni E., et al.: Pain prevalence, intensity, assessment and management in a hospitalized pediatric population. Pain Manage Nur. 2014;15:22-35
- Menezes M.S., Gozzani J.L.: Analgesia pós-operatória em pacientes pediátricos: estudo comparativo entre anestésico local, opióides e antiinflamatório não esteróide. Revista Brasileira de Anestesiologia. 2002;52:175-184
- Vittinghoff M., Lönnqvist P.A., Mossetti V., et al.: Postoperative pain management in children: Guidance from the pain committee of the European Society for Paediatric Anaesthesiology. 2018;28:493-506
- Walker S.M.: Pain after surgery in children: clinical recommendations. Curr Opinion Anaesthesiol. 2015;28:570
- Nowicki P.D., Vanderhave K.L., Gibbons K., et al.: Perioperative pain control in pediatric patients undergoing orthopaedic surgery. J Am Acad Ortho Sur. 2012;20:755-765.
- Kokki H.: Nonsteroidal anti-inflammatory drugs for postoperative pain. Pediatric Drugs. 2003;5:103-123
- Baer G.A., Rorarius M.G., Kolehmainen S., et al.: The effect of paracetamol or diclofenac administered before operation on postoperative pain and behaviour after adenoidectomy in small children. Anaesthesia. 1992;47:1078-1080
- Tawalbeh M.I., Nawasreh O.O., Husban A.M.: Comparative study of diclofenac sodium and paracetamol for treatment of pain after adenotonsillectomy in children. Saudi Medi J. 2001;22:121-123.
- Bhavsar M.M., Kheskani D., Shah P., et al.: A comparative evaluation of per rectal diclofenac sodium and paracetamol for postoperative analgesia in case of hydrocephalus. Int J Med Sci Public Health. 2015;4:373-376.
- Mireskandari S.M., Makarem J.: Effect of rectal diclofenac and acetaminophen alone and in combination on postoperative pain after cleft palate repair in children. J Craniofacial Sur. 2011;22:1955-1959
- Nnaji C.T., Onajin-Obembe B., Ebirim L.: The analgesic effects of rectal diclofenac versus rectal paracetamol following caudal-bupivacaine for pediatric day-case inguinal herniotomies: A randomized controlled prospective trial. J Pediatric Sur. 2017;52:1384-1348

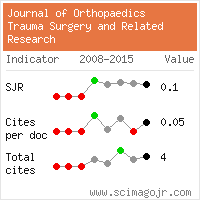
 Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.
Journal of Orthopaedics Trauma Surgery and Related Research a publication of Polish Society, is a peer-reviewed online journal with quaterly print on demand compilation of issues published.